Research
Mechanoelectrical transduction in hair cells
Electromechanical transduction in outer hair cells
Mechanoelectrical transduction in hair cells
Sound induced fluid vibrations in the cochlea are transduced into an electrical current by mechanosensitive ion channels located in the stereocilia. These channels are “gated” – opened and closed – as a result of deflection of the stereocilia. The mechanism for gating these channels is still unknown. Gating is purely mechanical. This is simply because of the requirement of extraordinarily high speed: 200 kHz in some bats and a “modest” 20 kHz in humans. Gating has been shown to be associated with extremely fine extracellular filaments, called tip links; their diameter is about 6 nm. Destruction of tip links, by for example sound overstimulation, leads to deafness.
We investigate mechanoelectrical transduction processes, employing the whole-cell patch-clamp technique in combination with fluorescent imagining of calcium uptake using a two-photon confocal laser-scanning microscope. We have shown that upon enzymatic or non-enzymatic destruction of the tip links, the channels appear to open, yielding a “tonic” current (Meyer et al., 1998). We showed that this tonic current is mechanosensitive and that the gating process is calcium dependent (Meyer et al., 2005). The data present evidence for an intracellular, calcium-dependent elastic gating element. Present experiments are concerned with location of the mechanosensitive channels and calcium dynamics in the stereocilia.
Electromechanical transduction in outer hair cells
A change of membrane potential of the outer hair cell (OHC) is transduced into a mechanical force, causing the cell body to contract and elongate. This electrically induced motion, called somatic electromotility, is believed by most researchers to be the basis of the exquisite frequency selectivity, sensitivity and dynamic range of the cochlea. The electromechanical force produced by the cell is thought to counteract damping forces in the cochlea, such as those inherent to the motion of fluids and cells, to produce the extraordinary mechanical tuning of the cochlea. The motor molecule responsible for somatic electromotility was cloned by the team of Peter Dallos; it belongs to a gene family, the solute carrier family 26, which encodes anion transporters and related proteins; it is called prestin. There are at least 6,000 of these molecules per square micrometer of cell surface. It is not known how the motion of all these molecules is coupled to the cytoskeleton, to generate the somatic electromotility. As with mechanoelectrical transduction in the stereocilia, the process must be exceedingly fast because it is required to work up to ultrasonic frequencies. Destruction of this transducer leads to deafness.
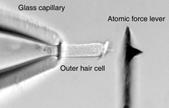
We investigate the electromechanical transduction process und employ laser Doppler vibrometry together with atomic-force spectroscopy. Using the vibrometer we showed that somatic electromotility can follow changes of the membrane potential cycle-for-cycle up to at least 100 kHz (Frank et al., 1999). We thereby established that the soma of the OHC has the necessary molecular machinery to generate electromechanical responses – with little attenuation and time delay – well-above the frequency limit of hearing. Since then we have developed an innovative method to measure the mechanical properties of biological structures up to at least 40 kHz (Scherer et al., 2000; Scherer and Gummer, 2004a). This technique employs an atomic force cantilever and has general applications outside the area of hearing research; it is particularly attractive for investigation of biological structures in a fluid.
We are applying the technique to investigate mechanical and cell biological properties of cellular and membranous structures in the cochlea. In collaboration with the research groups of Marlies Knipper and Dominik Oliver, it was recently shown that the somatic force generated by the OHC might be influenced by an interaction between prestin and a calcium/calmodulin-dependent serine protein kinase (CASK) in the basal cellular region where prestin expressing OHC membrane abuts prestin-free OHC membrane (Cimerman et al., 2013).
The requirements of high sensitivity, frequency selectivity and dynamic range can only be achieved by means of a mechanical amplifier in the cochlea. There are two schools of thought as to how the amplification might be achieved. Both hypotheses rely on the generation of mechanical force from an electromechanical transducer in the outer hair cells: one hypothesis maintains that the transducer is located in the soma and the other asserts that it is in the stereocilia. The soma hypothesis has the advantage that the outer hair cells are known to be electromotile over the entire functionally relevant frequency range (Frank et al., 1999), but the disadvantage that the receptor potential – the drive for the somatic electromotility – is low-pass filtered by the electrical impedance of the basolateral cell membrane (Preyer et al., 1996). Low-pass filtering, relative to stereocilia displacement, attenuates and delays the receptor potential to such an extent that somatic electromotility alone can not be responsible for cochlear amplification. The hypothesis that the relevant high-frequency electromechanical transducer is located in the stereocilia has the advantage that it does not suffer from low-pass filtering by the stereocilia membrane. However, it has the disadvantage that, to date, there is no direct experimental evidence that the stereocilia are electromotile at high frequencies. This having been said, there is evidence from the group of Robert Fettiplace that the stereocilia of outer hair cells possess electromechanical transducers. Whether these transducers can operate at sufficiently high frequencies and can inject enough mechanical force into the organ of Corti remains to be discovered.
We investigate mechanisms of cochlear amplification by the outer hair cells. Based on three-dimensional vibration measurements, we reported that the tectorial membrane presents resonant motion in the radial direction (Gummer et al., 1996; Hemmert et al., 2000a). We suggested that the temporal properties of this motion might compensate for the delay of the somatic electromechanical force produced by the electrical impedance of the basolateral membrane of the outer hair cell. These experiments gave the first hint as to how the electromechanical force might be coupled into the organ of Corti to yield cochlear amplification. Since damping and inertia of the organ of Corti might also cause severe attenuation and delay of the electromechanical force at high frequencies, we have also measured the mechanical impedance of the organ of Corti (Scherer and Gummer, 2004a). We found that for point loading, the organ of Corti moves like a purely viscoelastic material at all physiologically relevant stimulus frequencies; that is, there is no inertial component. Combining these impedance measurements with measurements of the velocity of the organ of Corti in response to intracochlear electrical stimulation, we found that the electromechanical force exerted at the reticular lamina has a broad resonant maximum (Scherer and Gummer, 2004b). We suggest that piezoelectric resonance in the outer hair cell, as proposed by Bill Brownell and colleagues, might be responsible for this force response. This resonance might compensate for the amplitude attenuation caused by the electrical impedance of the basolateral membrane of the outer hair cell.
We are now investigating how the electromechanical force is coupled to the stereocilia of the inner hair cells (IHCs). To date, we have demonstrated a new mode of stimulation of IHC stereocilia in response to the somatic electromechanical force produced by the OHC: Up to stimulus frequencies of at least 3 kHz for all cochlear turns, this force produces counter-phasic motion between the upper surface of the IHC and the lower surface of the overlying tectorial membrane (Nowotny and Gummer, 2006; Nowotny and Gummer, 2011). This vibration mode is additional to the (classical) shearing motion between the tectorial membrane and the upper surface of the hair cells. The frequency range is relevant for speech.
Although there are a large number of audiometric tests available for the differential diagnosis of hearing disorders, it is still not possible to locate the position of potential mechanical losses within the human cochlea. This is due to the fact that the inner ear is embedded in the temporal bone in humans and, therefore, not directly accessible for medical examination. Clinically, the only indicator of hearing impairment due to damage of the cochlear amplifier is loss of otoacoustic emissions. However, emissions do not allow a differential diagnosis of the cochlear amplifier; for example, to decide whether malfunction is due to decoupling of the stereocilia of the outer hair cells from the overlying tectorial membrane, or due to disruption of the motor complex in the basolateral wall of the outer hair cell, . . . etc.
As a starting point to develop a differential diagnostic of the cochlear amplifier, we have developed a laserinterferometric technique to assess non-invasively the mechanical function of the cochlea by performing vibration measurements of the ear drum. We have named the device a “Laseraudiometer”. We have re-designed the measuring system, that we developed earlier based on a commercially available laserinterferometer (Rodriguez Jorge et al., 1997), to enable vibration measurements near auditory threshold. Using our custom-made device, we have shown that otoacoustic emissions measured as vibration of the ear drum (at the umbo) can be used to: 1) provide an accurate estimate of auditory threshold (Dalhoff et al., 2007, Turcanu et al., 2009), and 2) estimate the mechanical parameters of the middle ear (Dalhoff et al., 2011).
Experiments are proceeding with the invaluable assistance of normal-hearing subjects and patients with sensorineural hearing loss.
Outer hair cells (OHCs), like many other polarized cell types, possess an endoplasmic network of tubules and vesicles. In general, it serves specialized functions, including protein synthesis, sequestration of calcium, production of steroids, storage and production of glycogen, and insertion of membrane proteins. This network appears to be more elaborate in OHCs than in other cells. The lateral cisternae are an integral part of the endoplasmic system of OHCs, and are involved in membrane turnover. Hair cells are known to have endocytic activity at both their apical (endocytosis) and synaptic (exocytosis) poles. The role of membrane recycling, and especially endocytosis, is not yet fully understood in OHCs.
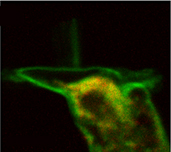
We are investigating membrane turnover processes. To investigate vesicle transport in OHCs we use various fluorescent dyes; for example, FM1-43 as a membrane marker, DiOC6 for endoplasmic reticulum and neutral-red as a lysosomal probe. Fluorescence is visualized with a two-photon laser-scanning microscope. FM1-43 reversibly stains the membrane; trapped vesicles can be visualized when the membrane is endocytosed. We have demonstrated endocytosis at the apical pole of OHCs; the destinations of endocytosed plasma membrane are the infracuticular zone, subsurface cisternae and the synaptic region (Meyer et al. 2001; Kaneko et al., 2006). We have shown that rapid endocytosis is a calcium/calmodulin-dependent process, with extracellular calcium ions entering through voltage-gated calcium channels at the subnuclear pole of the cell (Kaneko et al., 2006). The two distinct destinations of endocytosed membrane is consistent with the functional partition of the OHC: the basolateral wall for electromechanical transduction and the subnuclear pole for electrochemical transduction processes. The aim of present studies is to reveal the regulation of the intracellular traffic of trapped vesicles within OHCs.
Two-photon confocal
laser-scanning microscope
Estimation of middle-ear parameters using vibration otoacoustic emissions